Services is what make our business outstanding. We provide customers with optimal packaging solutions from specification, tests to validation and the actual supply of the material in full commercial scope: regulatory support, importation, payment, forecast, inventory planning and delivery.

-
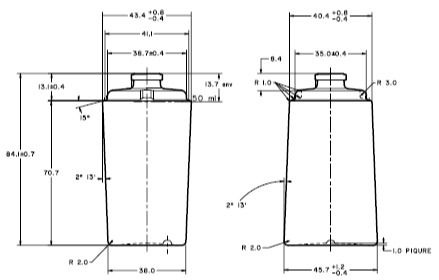
Product Specification
Product specification in any development can be a very lengthy process, we help our customers to shorten the process by our knowledge of the manufacturing process, the application technology, regulatory requirement, functionality & marketing concerns.

-
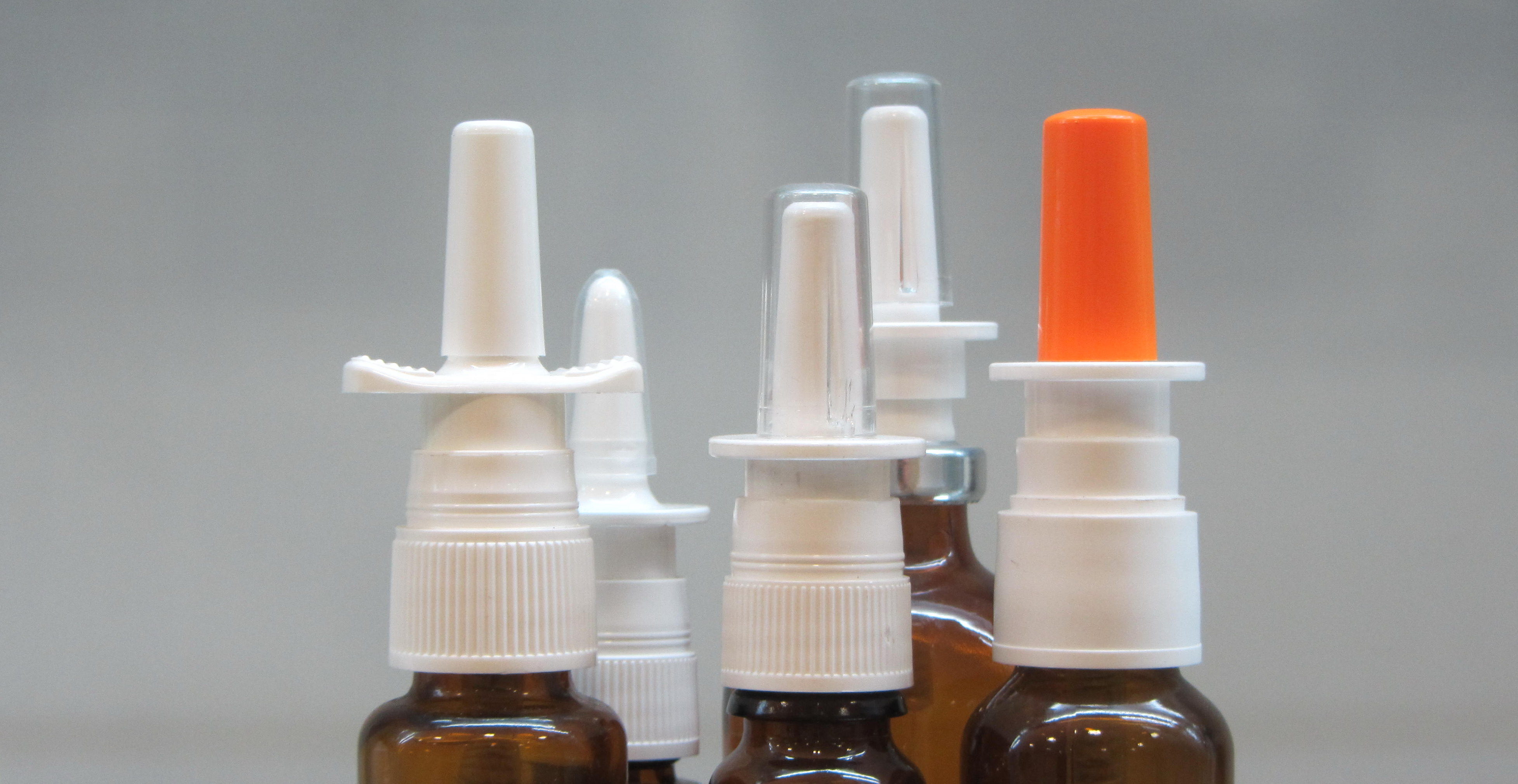
Quality Agreement
Balancing the interest of the users and the manfacturers is always a challenges facing any quality negotiation. We mediate professionally to seek the common goals that befit both party's imperatives in high standard and compliance.

-
Contract Support
As a distributor, we tailer made variety of offers. Various or combination of deals, order planning, stock rotation, payment schemes. All these has the only objective to make sustainable business that foster long term partnership.

-
Stock planning
Lean management gives great pressure to any company who must face challenge in tight cash flow, long leadtime, JIT requirement. We work closely with our clients to work out seamless supply plan to enhance smoothness and security.

-
Import & Forex
Country like China, forex and import hassle extinguishes the interest to buy imported goods. We are expert in this area to deliver the goods and services in wholesome integrity to our clients' door step as if they buy locally.


-
Quality Management
Certificates on International Accreditation on Quality Management such as ISO 9001, GMP, ISO 15378, ISO 13485, HACCP, ISO 14644-1 etc. of our representation are available on request.


-
Industrial Certification
Product or Production Qualification by ISO or Industrial standard such as Cleanroom Class 7 & 8, DIN EN ISO 8317 Child-resistant packaging, ISO 13485 for medical devices, etc. of our representation are available on request.

-
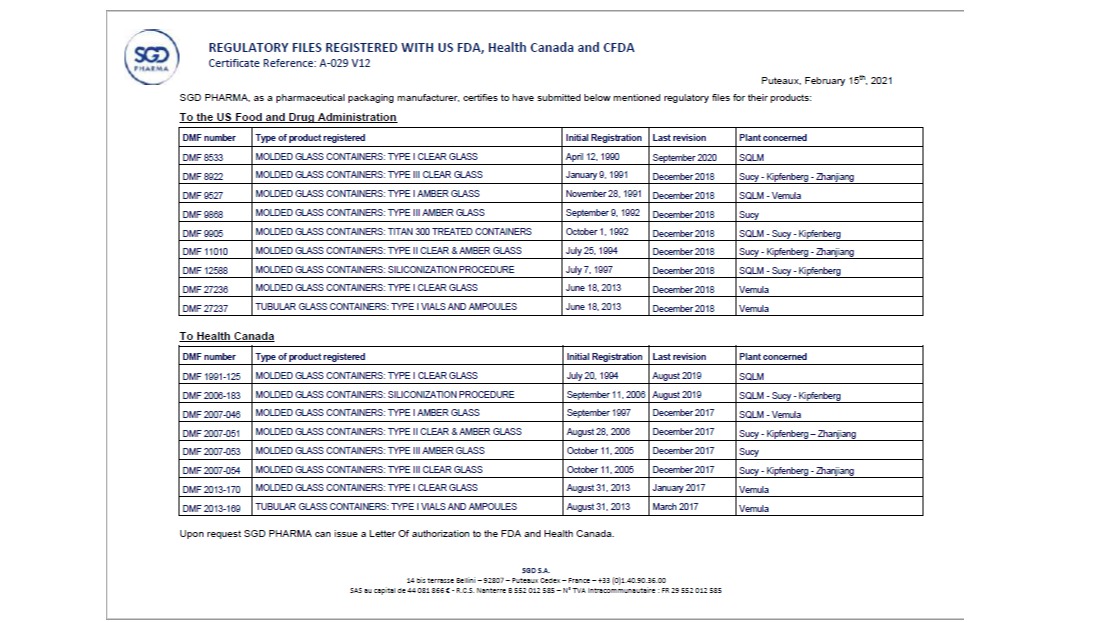
DMF/ LOA; CDE/ LOA
Many of our products are DMF registered in FDA/ Canada Health and CDE/ China. Customers can request LOA for their regulatory related process.


-
Manufacturers' Certificates
Certificates of Analysis, Certificate of Compliance, Quality Certificates of any sold products as document accompanying a shipment. Other manufacturers' certificates can be available upon request.


-
MSDS
MSDS, safety and medical use information on raw material, master batch can be available upon request.
-
Lab Test Certificates
We can also supply Certificate on batch analysis by external lab. We do annual lab tests against YBB standards for all CDE registered items.


-
Compliance information
Compliance information can be available as certificates, statement of declaration, validation reports, and thirt party audit information. It can be production or product qualification, safety or any topic like DEHP, Nitrosamine, or a test SOPs, ASTM, ISO, USP, etc.


-
Registration CDE/China
We register all our primary packaging material with CDE for as early as CFDA set up the platform. The task is still going on year by year for new products registration, and we maintain the files active and update annually. We manage also changes to the CDE files and give support to affected clients for the Change.

-
Registration FDA/ EU/ Canada
Our products are also DMF in FDA/ Canada/ EU, the task is still going on for new product registration. DMF is maintained active and updated. LOA can be issued upon requested.

-
Plant Audit
Customers who want to audit the manufacturers by their own QA or by third party auditors can be arrangement according to set protocol. We will render assistance to the logistic, and whatever follow up actions and communications needed.


-
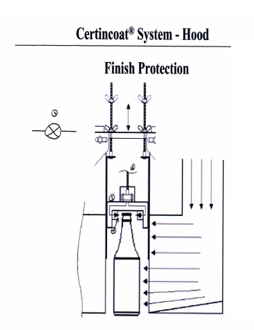
Surface Treatment
On finished glass container, we can further treat the bottles for certain desired effect:
Hot Treatment
Hot treatment on extermal surface of the glass vials: it is an external spraying of SnO2 to impart extra strength to the glass bottles against abraision, vibration, shocks and stress from logistic handling and pharmecutical process. It reduces breakage and bump checks. The spraying is done at hot end in the glass manufacturing process, it is then call Hot Treatment.
Cold Treatment
Cold treatment on external surface of the glass vials: it is an external spray coat of non ionic surfactant: SIMULSOL, α-glycols, PE monostearate. It enhances the gliding property of the glass surface, so that the glass vials can resist stress from squeezing and squambling on feeding line, turning table. Reduce breakage and bump checks. The spraying is done just after the annealing lehr closed to the cold end of the glass manufacturing process, it is then called Cold Treatment.
-
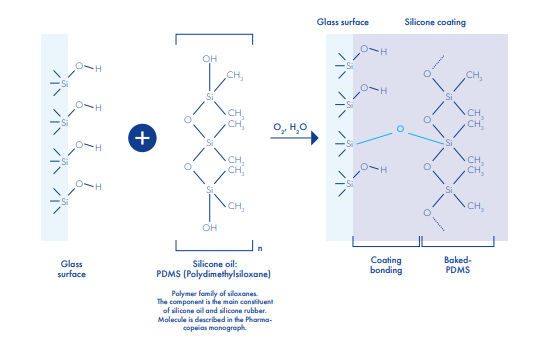
Siliconisation
Siliconization is realized with an aqueous emulsion of a polydimethysiloxanic oil, conforming to all pharmacopeias, sprayed inside the vials. The silicone film is fixed onto glass through the annealing lehr. All vials from 5ml to 1L, clear or amber, Type I, II or III glass may be siliconized. To maintain the quality of the coating, siliconized vials should be stored in dry condition and at moderate temperature. Silicone breaks down at temperature over 350° C.
Major qualities products for pharmaceutical:
It provides a protective layer useful for fragile molecules, further minimizing the interactions between the product and the container- It prevents high viscosity infusions from sticking to the internal surface of the vials giving them a perfect transparency
- Finally, due to its hydrophobic properties, the silicone coating ensures a higher restitution rate down to the last drop of product
- Compatibility tests between the product formulation and the siliconized vials have to be performed. The resistance of the silicone coating depends on the chemical structure of the pharmaceutical product, as well as its dissolving power on silicone.

-
Plastic coating
Plastic coating Technical support is available in many forms as part of the sales services such as specification development, product or process validation, on-site visit by our technical staff, technical seminars and laboratory support.


-
Depyrogenation
Depyrogenation Technical support is available in many forms as part of the sales services such as specification development, product or process validation, on-site visit by our technical staff, technical seminars and laboratory support.

-
ETO/ gamma sterilisation
ETO/ gamma sterilisation Technical support is available in many forms as part of the sales services such as specification development, product or process validation, on-site visit by our technical staff, technical seminars and laboratory support.


