Packaging that comes in contact with pharmaceutical product is an integral part of that product as their compatibility is essential to drug safety. Packaging materials must be traceable from recognized origins to conform to the most stringent regulatory requirement. The primary packaging we offer also concerns the functional aspect of how a drug product is to be used or administered.
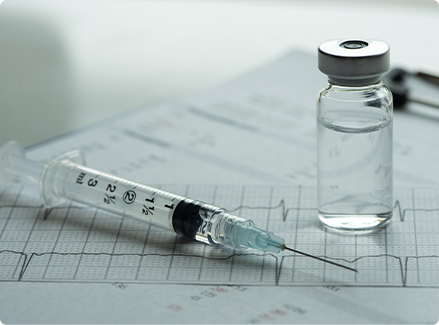
Parenteral packaging must have the correct chemical profile to minimize interaction with the drug. It must be free from delamination, internal stain, small particles and cracks
Injection vials ‧ Injection bottles ‧ Lyophilisation bottle ‧ Ampoule ‧ Injection vials tubular Show More
Major considerations in product packaging are given to how patients use the drug and the security features: Tamper-evident closure, CRC, closures with dropper pipettes, dosing aids, different sealing material and its sealing means
Syrup bottles ‧ Tablet bottles ‧ Tamper-evident closures ‧ Child-resistant closures Show More
A large portion of drug is effectively administered through our skin. Packaging considerations for topical drugs must be given to how they be applied onto the skin, the hygiene and the ease of application
Syrup bottles ‧ Glass jars ‧ Medicinal Oil ‧ Sprayer Dispenser ‧ Sprayer Fine mist Show More


